Return to: U of M Home
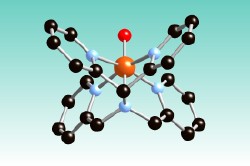
Que Group: The Que group focuses on bio-inorganic chemistry, specifically on the topic of iron, oxygen and biocatalysis. Our research efforts, involving a combination of biochemical, synthetic inorganic, and spectroscopic approaches, are aimed at understanding the oxygen activation mechanisms of nonheme iron enzymes, designing functional models for such enzymes, trapping and characterizing reaction intermediates, and developing bio-inspired oxidation catalysts for green chemistry applications. Targets include methane monooxygenase and related diiron enzymes, as well as monoiron enzymes with a common 2-His-1-carboxylate facial triad active site, including enzymes that require alpha-keto acids as co-substrates, Rieske dioxygenases that carry out cis-dihydroxylation of arene double bonds, and a novel pair of Fe- and Mn-dependent catechol dioxygenases. Intermediates of prime interest are high-valent oxoiron species thought to be carry out the most challenging oxidative transformations. The picture shows the crystal structure of an oxoiron(IV) complex we have characterized, such as the hydroxylation of methane and the conversion of water to O<sub>2.
 |
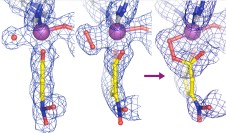
Lipscomb Group: We study the mechanisms used by metalloenzymes to activate molecular oxygen. The specific enzymes we study fall into two broad classes. Some catalyze incorporation one or both atoms of oxygen from dioxygen into their organic substrates (methane monooxygenase, aromatic ring cleaving dioxygenases, or cis-diol forming Rieske dioxygenases). The second class create an in situ reagent from the activate oxygen for biosynthetic purposes without oxygen incorporation (isopenicillin N synthase, fosfomycin synthase, ACC oxidase). We utilize a variety of techniques including EPR spectroscopy, transient kinetics, crystallography, and diagnostic substrate reactions. One of our main goals is to discover the intermediates in the chemical reaction cycle of oxygen activating enzymes. These efforts have led to the discovery of intermediates in each of the enzyme classes listed above, most recently culminating in the solution of the crystal structures of the key intermediates in the extradiol aromatic ring cleaving dioxygenase class (Figure below).
 |
Tolman Group: The goal of our research in bioinorganic chemistry is to gain a fundamental structural, spectroscopic, and mechanistic understanding of metalloprotein active sites of biological and environmental importance via the synthesis, characterization, and examination of the reactivity of model complexes. Specific projects are currently focused on understanding copper-dioxygen reactivity relevant to copper oxygenases and oxidases, characterization of novel reactive copper-oxygen species, and copper–sulfide centers in various copper proteins. For details, click here.
 |
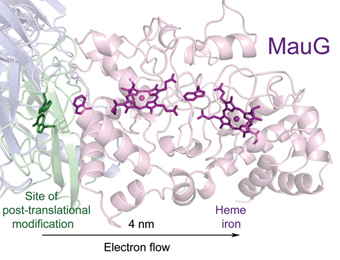
Wilmot Group: In the Wilmot lab we seek to understand the structural basis of catalysis by metalloenzymes. The principle tool of our research is macromolecular X-ray crystallography, in combination with spectroscopic techniques both in the crystal and solution, kinetics, mass spectrometry and mutagenesis. We freeze trap catalytic intermediates in the crystal, which give structural "snapshots" along the reaction pathway. These are then assembled into a "movie of catalysis" at the molecular level. We work on MauG (a di-heme enzyme that is involved in the synthesis of the protein-derived cofactor, tryptophan tryptophylquinone [TTQ]); chlorite dismutase (a key enzyme in bacteria mediated chlorite breakdown in water treatment facilities); nickel homeostasis in bacteria (a potential drug target).
 |
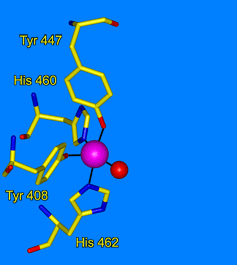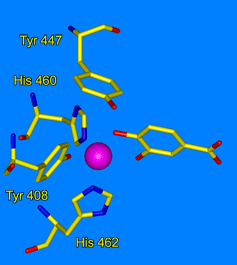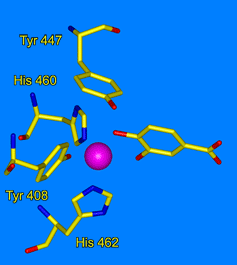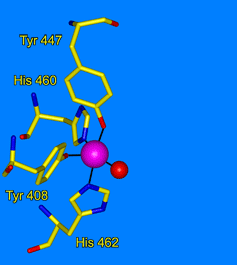
Ohlendorf Group: Our lab uses crystallography to understand the structural foundations of biological activity. Our study of metalloenzymes focuses on the nonheme catecholic dioxygenases. These enzymes have a long and rich history due to their ease of purification and variety of applicable spectroscopic tools. We use site-directed mutagenesis to probe specific residues in the active site. We use selection and directed evolution to develop new activities toward alternate substrates. We study the catalytic process through looking an anaerobic complexes with alternate substrates, by freeze trapping intermediates in slowed reactions, and by using Laue techniques to watch reactions with nanosecond resolution. We also use neutron diffraction to directly observe the protonation state of residues and substrates during catalysis.
 |
Pierre Group: Research in the Pierre Group uses an interdisciplinary approach toward the development, validation, and promulgation of metal-based probes for biological and medical applications. We are currently developing luminescent lanthanide-based sensors for the ratiometric detection of small molecules and ions of biological importance. Of current interest are reactive oxygen species (hydroxyl radical, singlet oxygen and nitric oxide), physiological cations (potassium, sodium, and lithium), and anions (phosphates and nucleotides). The very long lifetime of these complexes, and their selective turn-on luminescence upon binding or reacting with the desired analyte, renders them promising candidates for the quantitative spatial and temporal detection of their target. Similarly, we are developing the first ratiometric responsive contrast agents for Magnetic Resonance Imaging based on paramagnetic, lanthanide-based fluorine probes. The development of this new class of responsive MRI contrast agents could rapidly be expended to the selective in vivo detection of any biomedical reporter for both biomedical research and diagnosis purposes.
 |
Hooper Group: We study the structure, mechanism and evolutionary history of the metallo-enzymes which oxidize ammonia to nitrite in the chemolithoautrophic bacterium, Nitrosomonas. The hydroxylamine-oxidizing enzyme contains 8 covalently bound c-hemes; the catalytic c-heme is also covalently crosslinked to a tyrosine. The enzyme shares ancestry with a penta-c-heme nitrite reductase lacking the tyrosine crosslink. A tetraheme c-cytochrome has a pentacoordinate heme in a putative ubiquinone binding site. In a new family of beta-sheet mono-heme c-cytochromes, a putative N-oxide dehydrogenase, cytochrome P460, has a covalent lys crosslink to the catalytic heme; the enzyme is closely related to a beta sheet cytochrome c’ lacking the lys crosslink.
Lu Group:We seek to unravel how metalloenzymes convert small molecules using inorganic chemistry. Our approach is to develop, investigate, and exploit unusual structure-function motifs that are found in nature. For example, Fe-Fe hydrogenases may utilize metal-metal bonding for activating hydrogen. By preparing model coordination complexes featuring reactive metal-metal bonds, we can extract a detailed picture of their physical and electronic structures that may elucidate the underpinnings of their function. We can then apply this understanding towards new systems that improve on the natural counterparts in activity, selectivity, and substrate scope.