Environmental Solid State Chemistry - Nanocystalline Materials - Geochemical Cycling
| Research | People | Publications | Vita | Teaching | Characterization Facility |
Research in the Penn Group
The Penn group works in four major areas:
1) Elucidating the fundamental aggregation and growth mechanisms - especially nonclassical crystal growth mechanisms - of inorganic nanoparticles.
2) Characterizing the reactivity of natural and synthetic nanoparticles in environmentally relevant conditions.
3) Characterizing the magnetic behavior of iron oxide nanoparticles (e.g., natural and synthetic ferrihydrite, goethite, and magnetite).
4) Designing and implementing effective curriculum to strengthen and improve middle school and high school students’ understanding of science and engineering.
1) Elucidating the fundamental aggregation and growth mechanisms - especially nonclassical growth crystal mechanisms - of inorganic nanoparticles.
Work in the Penn group focuses on the fundamental growth mechanisms of a wide range of environmentally and technologically important nanomaterials, with the goal of controlling particle size and shape, phase and composition, the distribution of elements throughout each particle, and defect concentration. Achieving control over size, morphology, and microstructure of growing nanoparticles is a longstanding goal in the fields of materials chemistry and colloid chemistry. We focus on a range of crystal growth mechanisms, including growth in confinement, oriented aggregation, and classical crystal growth mechanisms such as coarsening.
Current materials of interest include iron oxides and oxyhydroxides, zinc oxide, titanium dioxides, cobalt oxyhydroxide, and several other transition metal oxides and sulfides. In addition, we have ongoing collaborative projects looking at greener materials for use in sustainable energy applications (e.g., lead-free perovskites for the absorber layer in a solar cell). We also enjoy several productive and exicting collaborations with researchers from around the country. For example, we have worked with the McCormick group on silver nanostructures, the Holmes group on perovskites, and the ICDC (Inorganometallic Catalysis Design Center) on catalytically active metal organic framework materials.
We use several materials characterization methods, including high-resolution transmission electron microscopy (HRTEM), cryogenic transmission electron microscopy (cryo-TEM), liquid cell TEM, X-ray diffraction (XRD), small angle X-ray scattering (SAXS), dynamic light scattering, among others.
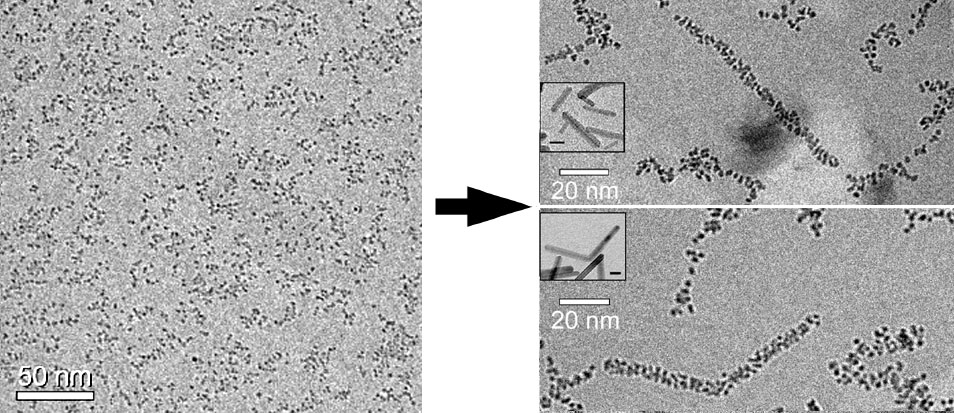
Dr. Virany Yuwono characterized, for the first time, intermediates of the crystal growth mechanism known as oriented attachment (Yuwono et al., 2010). The above images are cryo-TEM images of vitrified aqueous suspensions of iron oxide nanoparticles. On the left is the initial sample of ferrihydrite nanoparticles in vitrified water. On the right are vitrified samples after aging at elevated temperature. In these two images, the intermediates are seen - in twinned configuration in the lower image. These intermediates are composed of goethite nanocrystals, which are crystallographically aligned with respect to one another. The smaller insets show TEM images of the goethite crystals at a later stage. A major goal in the Penn group is to control this crystal growth mechanism so as to produce nanocystals of narrow size and shape distribution.
2) Characterizing the chemical reactivity of natural and synthetic nanoparticles in environmentally relevant conditions.
Nanoparticles are common, natural components of the Earth’s surficial materials, are increasingly found as anthropogenic contaminants in the environment, and are often employed in water treatment systems and engineered systems to remediate pollutants in groundwater. Processes like dissolution, precipitation, growth, electron-transfer reactions, and phase transformations strongly influence the fate and transport of a wide range of natural and anthropogenic chemical species in aqueous systems, and many of these processes occur at the surfaces of or resulting in the production or transformation of nanophase materials.
Our well characterized, synthetic materials are used in experiments aimed at elucidating the link between the physical (e.g., size and shape) and chemical (e.g., doping and chemical composition) properties and chemical reactivity. Much of our work in this area has focused on the iron-breaing minerals, which have been implicated as the dominant class of materials responsible for the abiotic degradation of contaminants in soils and sediments, and zero valent iron particles, which are currently employed in the field for remediating contaminated sites. Further, natural materials are used in parallel experiments so as to enable meaningful comparisons between the natural and model materials.
In addition, we work with several research groups in connection with the ICDC (Inorganometallic Catalysis Design Center) on catalytically active metal organic framework (MOF) materials. We develop synthetic methods for reproducibly preparing MOFs that are phase pure and with controlled size (tens of nm to microns). Catalytically active metals and bimetallics are then installed, with the aim of producing catalysts for the controlled oxidation of gases like methane (e.g., partial oxidation to methane). Materials are subjected to the catalytic conditions of interest. Small changes in particle size and phase purity can mean big changes in catalytic activity. Finally, we perform extensive materials characterization after syntheses, after installation of the active sites, and after use in the catalytic conditions of interest. The collaboration includes both computational and experimental projects, and the Penn group contributions are largely experimental.
We use HRTEM and other methods to carefully characterize solid-state changes resulting from reactions with both natural and anthropogenic chemicals so as to quantitatively assess reactivity, reactive surface area, and how reactivity evolves as reactions proceed. This last point is an important one since materials are expected to change substantially with time.
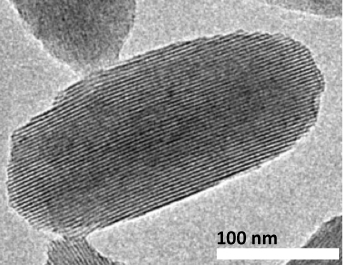
High resolution transmission electron micrograph of an NU-1000 nanocrystal.
3) Characterizing the magnetic behavior of iron oxide nanoparticles (e.g., natural and synthetic ferrihydrite, goethite, and magnetite).
We have the wonderfully good fortune of collaborating with Profs. Subir Banerjee and Josh Feinberg (Geology and Geophysics) on the fundamental magnetic properties of iron oxide nanoparticles. Our overarching goal is to build a more comprehensive foundation of our understanding of the initial state of nanoparticle iron ‘oxides’ and then follow, step-by-step, the abiotic iron reduction process. Magnetism is extremely sensitive to small changes in iron oxides as a function of continued reaction and offers to potential to better understand the solid-state changes in iron oxide nanoparticles during reactions important to, for example, the biogeochemical cycling of iron, contaminant degradation reactions, and even CLIMATE CHANGE!
4) Designing and implementing effective STEM programs and curriculum for middle school and high school students and teachers
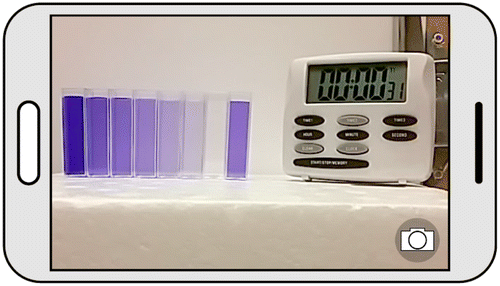
The Penn group has worked on a wide range of activities for use with both students and teachers. Microscopy Camp served seventh grade students and middle and high school teachers and focused on the particulate nature of matter, both light and electron microscopes, and fun science (including exciting demonstrations involving fire). One of Microscopy Camp's teachers, Eric Kehoe (Janesville Waldorf Pemberton High School) developed an idea that camera phones and other hand-held devices could be used to perform quantitative colorimetry analysis. Mr. Kehoe's students have come to the University of Minnesota to work with U of MN students applying the method. The Penn group is extending the method to a broad range of samples.
Check out these four publications!
A Fresh Look at the Crystal Violet Lab with Handheld Camera Colorimetry
Building a Successful Middle School Outreach Effort: Microscopy Camp
The Penn Research
Group receives gracious financial support from the following agencies:
The University of Minnesota
The National Science Foundation
The Department of Energy
The Department of Education
Nanostructured Materials and Processes (through IPRIME)
Environment and Natural Resources Fund (state of Minnesota)
Carestream Health, Inc.